
Untangling molecular crosstalk in the skin
Our team focusses on understanding the cellular and molecular crosstalk between keratinocytes- the epithelial cells of the skin- and their microenvironment in health and disease. During injury, fast repair mechanisms are critical to maintain the skin’s barrier function and we study how epidermal stem cells communicate with fibroblasts and immune cells to orchestrate inflammation and subsequent tissue regeneration. Wound repair can however predispose tissues to cancer initiation and we aim to identify novel molecular mechanisms through which inflammation, cell death and fibrosis modulate epidermal stem cells and impact on skin regeneration and neoplasia.
To dissect how intercellular crosstalk in the skin orchestrates inflammation, injury repair and/or tumour initiation, we are addressing four key questions:
1. What are the molecules that are released during cutaneous injury repair and how do they modulate regenerative and neoplastic responses?
2. Which molecular pathways control the activation of epidermal stem cells?
3. How do different modalities of keratinocyte cell death influence the skin’s tissue response?
4. Can we manipulate molecular signaling pathways in the skin to improve tissue regeneration, while preventing tumour initiation?
Areas of Expertise
- Mouse models of skin inflammation, regeneration and keratinocyte-derived tumorigenesis
- Innate immunity
- Epidermal stem cell assays
Technology Transfer Potential
- Novel treatments to improve chronic and diabetic cutaneous wound healing
- Identifying targeting strategies to prevent non-melanoma skin cancer
Selected publications
- Van Hove L. et al. Autophagy critically controls skin inflammation and apoptosis-induced stem cell activation. Autophagy (in press) (2023). Visit ➚
- Hoste, E. et al. OTULIN maintains skin homeostasis by controlling keratinocyte death and stem cell identity. Nat Commun 12, 5913 (2021). Visit ➚
- Van Hove, L. et al. Fibrotic enzymes modulate wound-induced skin tumorigenesis. EMBO Rep, e51573 (2021). Visit ➚
- Hoste, E. et al. Epithelial HMGB1 Delays Skin Wound Healing and Drives Tumor Initiation by Priming Neutrophils for NET Formation. Cell Rep 29, 2689-2701 (2019). Visit ➚
- Hoste, E. et al. Innate sensing of microbial products promotes wound-induced skin cancer. Nat Commun 6, 5932 (2015). Visit ➚
Bibliography
- Full bibliography Visit ➚
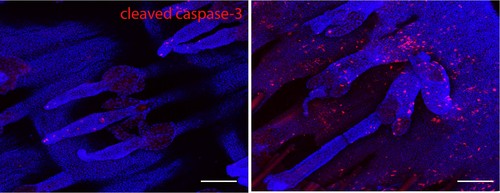
Image depicting tail wholemounts of normal (left) and inflamed (right) skin stained for cleaved caspase-3 (red) and nuclear counterstain (blue). A clear increase in apoptotic cells is observed in inflamed tail skin. Scale bar: 100 µm.

